Reteplase versus Alteplase for Acute Ischemic Stroke: A Journal Club Review
Shuya Li, Hong-Qiu Gu, Hao Li, et al. Reteplase versus Alteplase for Acute Ischemic Stroke. N Engl J Med. 2024; 390:2264-2273. DOI: 10.1056/NEJMoa2400314
BACKGROUND
Currently, alteplase and tenecteplase are the two fibrinolytic agents used in acute ischemic stroke within 4.5 hours after symptom onset. Recent studies have shown that tenecteplase delivers clinical benefits similar to those of alteplase, but only requires a single weight-based bolus, unlike the bolus plus infusion regimen required by alteplase. Despite having access to these two agents for acute ischemic stroke, there remains a demand for effective and affordable thrombolytic options.
Reteplase (Retavase), a recombinant plasminogen activator characterized by a double-bolus approach, was initially approved for the treatment of acute myocardial infarction. The results of this trial demonstrated that reteplase may be more likely to result in an excellent functional outcome compared to alteplase in patients with ischemic stroke treated within 4.5 hours of symptom onset.
What is the relevance?
Weight-based dosing may lead to medication safety errors as well as delay time to injection. Fixed bolus dosing, as used with reteplase, may enhance outcomes as well as safety in acute stroke situations.
GENERAL STUDY OVERVIEW
Trial design: Phase 3, multicenter, prospective, open-label, noninferiority, randomized trial with blinded end-point assessment
Objective: Compare reteplase to alteplase with respect to the functional outcome in patients with acute ischemic stroke
Funding: China Resources Angde Biotech Pharma and others
METHODS
Inclusion criteria
18 – 80 years old
Eligible to receive IV thrombolysis
Excellent functional status before onset of stroke (score < 1 on modified Rankin scale)
NIHSS score of 4-25
Exclusion criteria
Previously undergone or planned to undergo endovascular thrombectomy
Intracranial hemorrhage history or other bleeding tendencies
Interventions
1:1 ratio to receive IV reteplase or alteplase
Reteplase: 18 mg bolus followed by 18 mg bolus after 30 min
Alteplase: 0.9 mg/kg max of 90 mg
Primary end point
Excellent functional outcome on the modified Rankin scale of 0 or 1 at 90 days
Symptomatic intracranial hemorrhage within 36 hours
Secondary end point
Good functional outcome on the modified Rankin scale of 0 to 2 at 90 days
Early dramatic recovery with respect to the NIHSS score at 24 hours and at 7 days
Barthel Index score of at least 95 at 90 days
Statistical analyses
Estimated sample size of 1412 patients would provide 85% power at a one-sided significance level of 2.5%
Non-inferiority margin was set at 0.93
Intention to treat analysis
Superiority established if lower boundary of confidence interval was greater than 1
RESULTS
Participant Flow
1412 patients underwent randomization
707 patients assigned to reteplase
705 patients assigned to alteplase
Baseline characteristics
Baseline Characteristics were similar between both groups
Median age of 63 years old
70.5% male population
Median baseline weight ~68 kg
Median NIHSS score on admission was 6
Median time from stroke onset to administration was 180 min
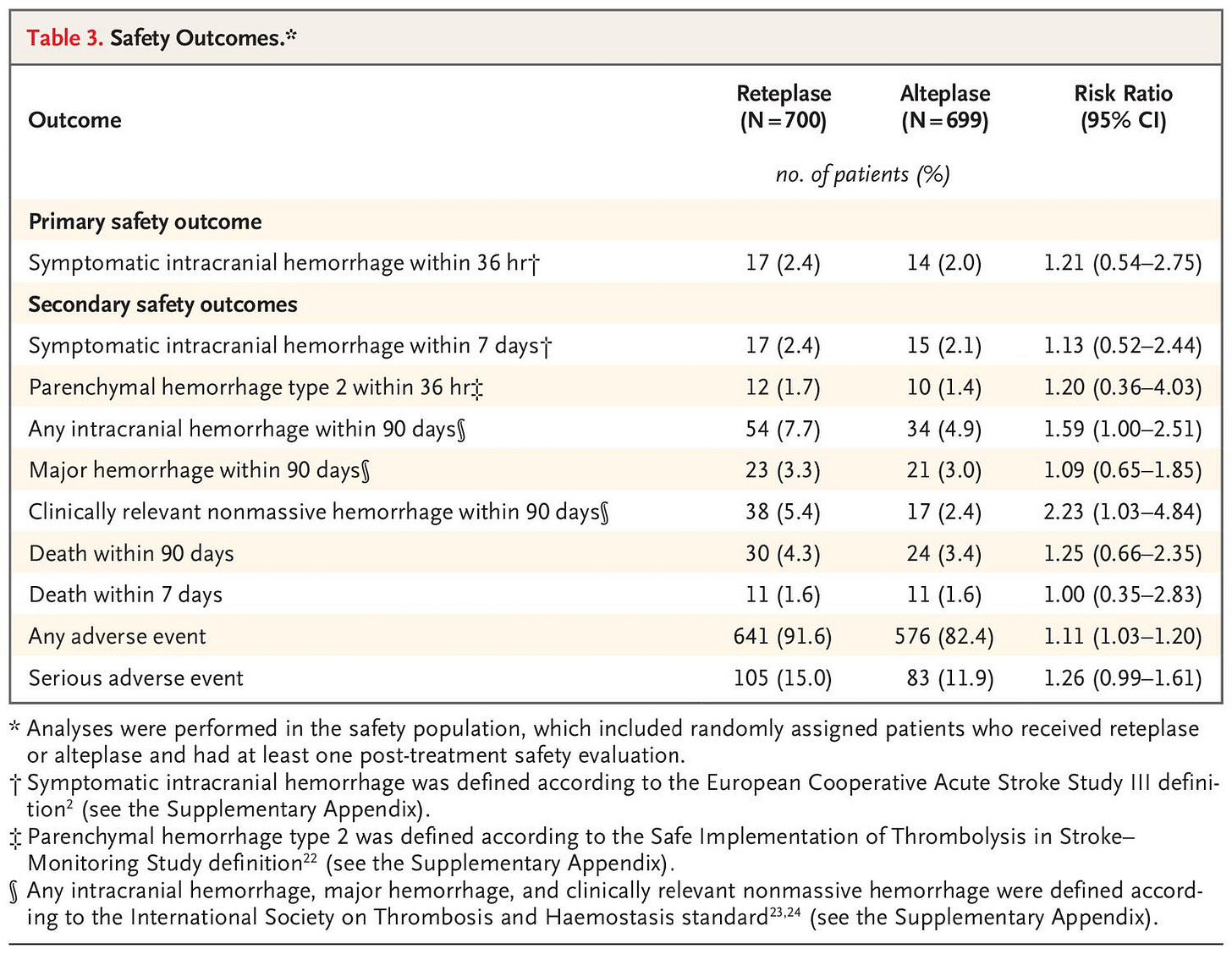
Study outcomes
Limitations
Open label
Non-generalizable patient population
Short term follow-up period
Low baseline weight in comparison to US
AUTHORS’ CONCLUSIONS
Among patients with ischemic stroke within 4.5 hours after symptom onset, reteplase was superior to alteplase with respect to an excellent functional outcome at 90 days. However, patients receiving reteplase had a higher incidence of any intracranial hemorrhage than those receiving alteplase.
Presenters’ Conclusion
With respect to the baseline characteristics of this study, reteplase is superior to alteplase at improving functional outcomes in acute ischemic stroke patients, however there may be a higher incidence of intracranial hemorrhage. Due to the lack of literature, I recommend not using reteplase until further studies are conducted to evaluate the safety and efficacy of tenecteplase vs reteplase.